Welcome to the Pepper Lab
Based at Brighton and Sussex Medical School (BSMS)
The University of Sussex, UK.
A Sussex based research team hunting for novel cancer therapies.
What we do
We are a multi-disciplinary team that combines clinical and scientific skills to understand disease processes and identify novel therapeutic targets.
Patients
to obtain tumour cells
Experiments
to study tumour cell behaviour and identify novel targets
Collaborate
to design and manufacture novel drug
Drug Development
to test novel drugs in cancer cell models
Patients
the aim of our team is to improve patient prognosis and treatment
Latest News
Lab Alumni
Projects
Stratifying and targeting apoptosis in diffuse large B-cell lymphoma (DLBCL) using a systems biology approach.
This PhD studentship is funded by a local philanthropist, Paul Stanforth. The project is designed to use NF-κB fingerprinting and link them to the expression of anti-apoptotic proteins to stratify diffuse large B cell lymphoma (DLBCL).
Stratification and selective targeting based on anti-apoptotic gene expression and NF-κB signalling in chronic lymphocytic leukaemia (CLL).
This PhD studentship is funded by a local philanthropist, Paul Stanforth. The project is designed to use NF-κB fingerprinting and link them to the expression of anti-apoptotic proteins to stratify chronic lymphocytic leukaemia (CLL).

Using NF-kB ‘fingerprints to identify therapeutic vulnerabilities within subsets of B cell malignancies
This exciting Blood Cancer UK funded project grant utilises the novel NF-kB fingerprinting technology developed by a collaboration between the Pepper and Mitchell team (www.mitchell.science) to predict the best drugs for patients with B cell malignancies.

In vitro modelling and therapeutic targeting of tumour cell migration in chronic lymphocytic leukaemia.
This programme continuity grant is funded by Blood Cancer UK. Tumour cells migrate to protective niches in the body to avoid destruction by conventional therapies. We are modelling this in the laboratory in order to identify the mechanisms tumour cells use to migrate.

Modelling and targeting Acute Myeloid Leukaemia cells in the bone marrow protective niche
This project grant is funded by a British Society of Haematology start-up grant and the Sussex Cancer Fund. Acute Myeloid Leukaemia is an aggressive disease with poor survival outcomes. The tumour cells remain anchored in the protective niche of the bone marrow where they can avoid destruction by conventional therapies.

Overcoming ibrutinib and venetoclax resistance in chronic lymphocytic leukaemia.
This project grant is funded by the Medical Research Council (MRC). Ibrutinib and venetoclax have revolutionised treatment of chronic lymphocytic leukaemia (CLL). However, they are non-curative and some patients remain refractory or develop resistance.

Preferential stem cell targeting using ProTide nucleoside analogues
This project grant is funded by the pharmaceutical company, Nucana. Although conventional anti-cancer approaches can frequently eradicate a large proportion of the bulk tumour, the most primitive stem cells are frequently chemo-resistant.
Featured Publications
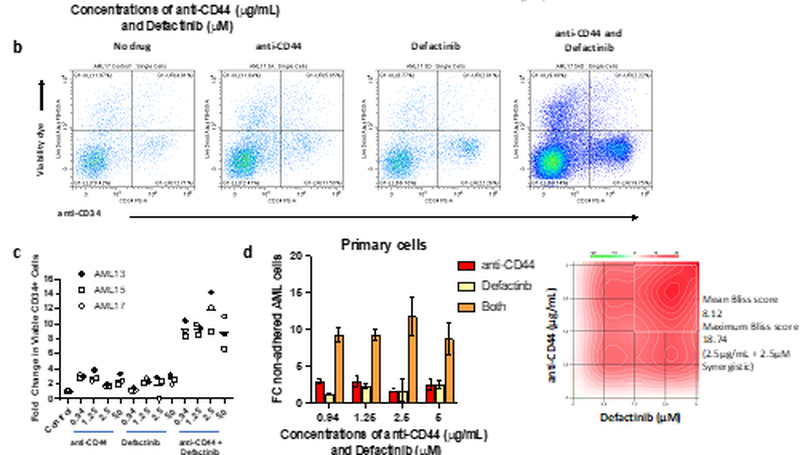
Adhesion of leukaemic cells in the bone marrow microenvironment (BMME), play an important role in the resistance of AML to current therapeutic agents. Although, therapies that disrupt AML cell adherence in the BMME, and release them into the less protective peripheral circulation, have been trialled (e.g. Plerixafor targeting CXCR4), their success has been limited. Here we report efforts to create a multi-cellular, physiologically relevant, in-vitro model of the adhesive and chemo-protective AML BMME. Firstly, we demonstrated that this model recapitulates the cell adhesion-mediated drug resistance (CAM-DR) seen clinically. Secondly, we used it to explore the altered transcriptional programme induced by cell adhesion and subsequently as a drug testing platform to rationally target and disrupt this cellular process to reverse the protective effects of the AML BMME. The key finding was that dual targeting of CD44 and FAK (using anti-CD44 and the clinical grade FAK inhibitor defactinib) synergistically inhibit adhesion of the most primitive CD34high AML cells that are associated with CAM-DR and relapse.
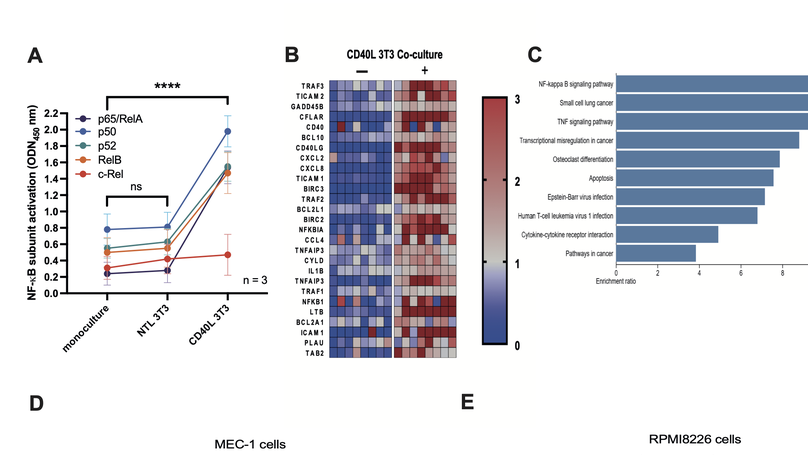
This study was designed to investigate the potential for targeting the NF-kB inducing kinase, NIK, in two common B-cell malignancies, chronic lymphocytic leukemia (CLL) and multiple myeloma (MM). Using a selective NIK inhibitor, CW15337, we were able to demonstrate that cell lines and primary tumor cells were sensitive to the effects of NIK inhibition, whilst normal lymphocytes were significantly more resistant to its cytotoxic effects. Sensitivity to CW15337 was associated with the nuclear expression of the NF-kB subunit, p52. Importantly, tumor samples from a subset of poor prognosis CLL patients, with mutations in a gene called BIRC3, showed elevated p52 expression and were particularly sensitive to NIK inhibition. Furthermore, the combination of CW15337 and ABT-199 (venetoclax) reversed the drug resistance observed when treating tumor cells with ABT-199 alone. Our study shows the potential for targeting NIK in both CLL and MM.
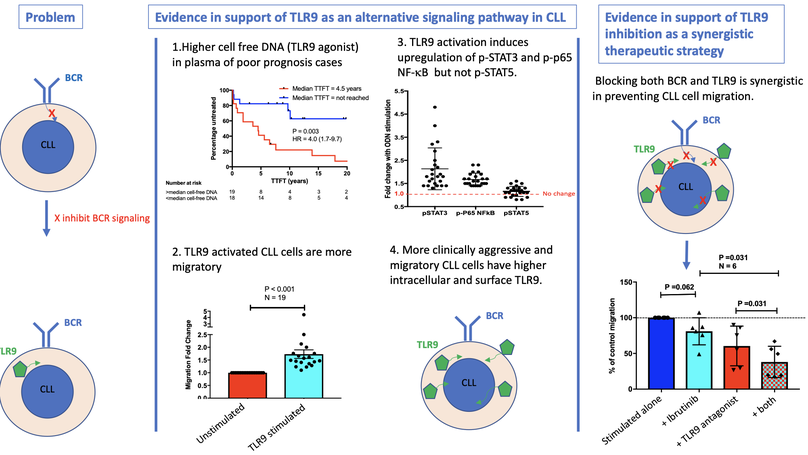
Chronic lymphocytic leukemia (CLL) remains incurable despite B-cell receptor-targeted inhibitors revolutionizing treatment. This suggests that other signaling molecules are involved in disease escape mechanisms and resistance. Toll-like receptor 9 (TLR9) is a promising candidate that is activated by unmethylated cytosine guanine dinucleotide-DNA. Here, we show that plasma from patients with CLL contains significantly more unmethylated DNA than plasma from healthy control subjects (P < .0001) and that cell-free DNA levels correlate with the prognostic markers CD38, β2-microglobulin, and lymphocyte doubling time. Furthermore, elevated cell-free DNA was associated with shorter time to first treatment (hazard ratio, 4.0; P = .003). We also show that TLR9 expression was associated with in vitro CLL cell migration (P < .001), and intracellular endosomal TLR9 strongly correlated with aberrant surface expression (sTLR9; r = 0.9). In addition, lymph node-derived CLL cells exhibited increased sTLR9 (P = .016), and RNA-sequencing of paired sTLR9hi and sTLR9lo CLL cells revealed differential transcription of genes involved in TLR signaling, adhesion, motility, and inflammation in sTLR9hi cells. Mechanistically, a TLR9 agonist, ODN2006, promoted CLL cell migration (P < .001) that was mediated by p65 NF-κB and STAT3 transcription factor activation. Importantly, autologous plasma induced the same effects, which were reversed by a TLR9 antagonist. Furthermore, high TLR9 expression promoted engraftment and rapid disease progression in a NOD/Shi-scid/IL-2Rγnull mouse xenograft model. Finally, we showed that dual targeting of TLR9 and Bruton’s tyrosine kinase (BTK) was strongly synergistic (median combination index, 0.2 at half maximal effective dose), which highlights the distinct role for TLR9 signaling in CLL and the potential for combined targeting of TLR9 and BTK as a more effective treatment strategy in this incurable disease.
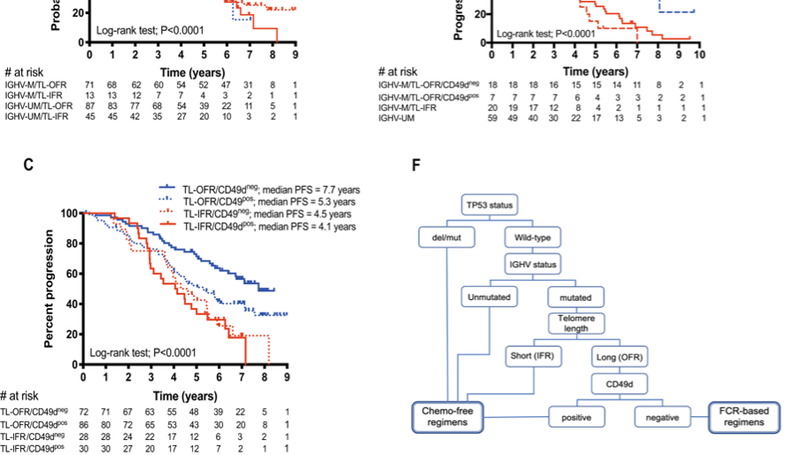
Combined analyses of the pairs of biomarkers A IGHV mutation status and CD49d, B IGHV mutation status and telomere length and C telomere length and CD49d as predictors of progression-free survival (PFS) in CLL patients. D Shows the overlaid Kaplan–Meier curves for the ARCTIC/ADMIRE cohort, which demonstrate that patients with mutated IGHV genes and short telomeres have a similar, inferior PFS to the unmutated IGHV subset. Furthermore, patients with mutated IGHV genes, long telomeres and low CD49d expression have a significantly longer PFS than patients with mutated IGHV genes, long telomeres and high CD49d expression. E An additional cohort, derived from the FC-treated arm of the UK CLL4 trial, confirmed the findings from the ARCTIC/ADMIRE cohort. F Shows a schematic diagram of the propose a risk-adapted approach to treatment selection, which would contra-indicate frontline chemoimmunotherapy for ~83% of patients.
Recent Publications
For links to all the recent publications from the team please click on the links below:
Contact
- C.Pepper@bsms.ac.uk
- Medical Research Building, University of Sussex, Falmer, East Sussex BN1 9PX
- Enter the Medical Research Building (note this is behind the Medical Teaching Building) and call reception. We are on the second floor, up the stairs through the security door.
- A.Pepper@bsms.ac.uk
- Chris's Twitter
- Andrea's Twitter
- Chris's BSMS Page
- Andrea's BSMS Page
- Chris's ORCID iD
- Andrea's ORCID iD












